general questions
1. What is SAVD?
SAVD stands for Saliva Amplification Viral Detection and is a CE-IVD-approved RT-PCR Covid-19 test. According to the Norwegian authorities, PCR as an analysis method is the recommended laboratory method for detecting genetic material from the coronavirus. The WHO defines this molecular diagnostics as the gold standard, as it is the most effective and reliable way to detect the presence of SARSCoV-2. SAVD confirms or denies the presence of SARS-CoV-2 RNA in 30-50 minutes (depending on machine and number of cycles), and the many properties of the test provide significant time and cost savings.
2. Is SAVD approved in Norway?
Yes. SAVD is a PCR test that is CE-IVD approved. This means that the test is approved for sale and use in accordance with European and Norwegian regulations. In other words, SAVD meets the Norwegian authorities' requirements for use.
3. What are the benefits of SAVD?
- One of the fastest PCR tests aimed at SARS-CoV-2 on the market, where the result is available within 30-50 minutes (depending on the machine and the number of cycles).
- Testing is user-friendly. SAVD is validated for sampling and collection of sample material from deep throat, anterior nose and deep nose, as well as a combination of these. It is up to the individual customer to assess where it is appropriate for their work to take the sample material from. Click here: See validation.
- SAVD targets the Orf1ab gene (the replication gene that encodes RNA replication of the virus), so that the mutated variants of SARS CoV-2 can be identified.
- A unique method that does not require RNA isolation and thus no need for RNA isolation equipment.
- Works with most open RT-PCR machines.
- SAVD is verified with specificity of 100 % and sensitivity of 99 % and can detect up to 20 virus copies per ml.
- SAVD comes pre-integrated with the award-winning Yoti digital identity app. Yoti enables test results to be handled in accordance with privacy laws, and is sent securely to mobile phones as a test certificate for uncomplicated confirmation of infection freedom and reduction of quarantine time.
- The SAVD components come freeze-dried. This makes transport and storage of the product easy.
- SAVD has been added with a deactivating enzyme which eliminates the risk of infection for the test staff.
4. Why does the WHO define PC (polymerase chain reaction) as the diagnostic gold standard for the detection of Covid-19?
Because PCR is a test that detects nucleic acids and increases the number of copies of the genetic material for easier detection. The test increases the amount of viral genetic material to several million copies so that the response results are more accurate and reliable. SAVD can detect the presence of Covid-19 with a minimum of 20 virus copies per ml.
5. What are the details of the technology used in SAVD?
SAVD is a PCR test consisting of a patented enzyme encoded against the ORF1ab gene by SARS CoV-2. To date, all versions of the virus have been detected with mutation of different segments on the S gene, so that SAVD also detects these. According to WHO guidelines, virus detection with one gene is standard during an ongoing pandemic, ie when there is high circulation. Furthermore, in a post-pandemic everyday life with lower viral circulation, virus detection with two genes is recommended.
The SAVD + test is under development. This is a further development of SAVD aimed at two genes; Orf1ab and E. SAVD + are used with the same equipment as SAVD.
6. Why is RNA not isolated, and what does this mean?
SAVD does not require RNA isolation because a special buffer composition, and therefore the analysis time is significantly shortened. In a normal RT-PCR test, the sample's buffer maintains an intact virus before an RNA isolation process. That is, the RNA is encapsulated in a protein coat, in contrast to SAVD's buffer, which destroys the virus's protein coat to make the RNA available for direct analysis. This is the fundamental difference in the analysis method for SAVD compared to traditional PCR analysis. This is both time- and cost-saving.
7. How are the samples analyzed?
The test is easy to perform and can be run on open traditional RT-PCR machines with fluorescein. With a typical RT PCR machine, for example Bio-Rad CFX 96, you can take up to 94 tests simultaneously. Due to the innovative technology that results in a simpler analysis method, SAVD can also be performed on smaller and mobile machines, as well as large machines with a high analysis volume. This range of analysis options means that the tests can be taken directly where the need is - in arrival halls, departure halls, by the street, before major events or by mass testing such as at border crossings or large facilities.
8. Can SAVD be used on any open PCR / qPCR machine?
Yes, SAVD is designed to be used on most standard open RT-PCR machines with fluorescein analysis. This is a conscious choice by the manufacturers, so that the users of SAVD are not bound to buy expensive PCR machines belonging to a specific supplier with systems linked exclusively to their own products. In a period where the pandemic has significantly increased the prices of PCR machines, SAVD enables the purchase of less expensive machines that are tailored to meet the needs of the individual (e.g. high volume/high number of wells vs. lower volume/lower number of wells). Examples of machines that can be used in connection with the SAVD test are MyGo Pro and Bio-Rad CFX96. The manufacturer of SAVD will have self-produced machines with different volume capacities available for sale during 2021.
9. Why is SAVD particularly suitable for stepping up to massive testing?
SAVD is particularly suitable for mass testing, both in terms of the test's unique characteristics, but also availability. As both developer and manufacturer of the patented active substances in SAVD, GeneMe does not have supply problems of tests or equipment, which is a growing problem nationally and internationally. The active ingredients in SAVD are produced using biotechnological methods in microscopic organisms that have been genetically modified by GeneMe, so that we as a supplier have unlimited access.
10. How can the individual obtain their test result?
SAVD also differs from other tests in terms of functionality. The results from the SAVD test can, if desired, be read via the award-winning digital identity app YOTI. YOTI makes it possible to handle test results in accordance with the privacy laws, GDPR. The result of the test is securely sent to the individual's mobile phone. The app is then used as an approved corona certificate. This means that the tests can easily be used for optimal logistics for e.g. border crossings, arrival and departure halls or similar. The test result will thus physically follow the person who has been tested, and will further enable great security for infection-free travel in everyday life. We point out that Yoti is an option for identifying the person being tested, but the test can also be used without the app.
11. What does SAVD consist of, and how does it work in practice?
SAVD consists of three main components; one set for physically taking the sample from the patient, one set for transferring the sample material to the samples to be run in the machine, and one set for running the samples in the machine. Everything comes complete in the same delivery. The only addition is the analysis machine itself. In each SAVD delivery package there are 32 tests, of 8-well plastic strips x 4. As a safety mechanism, the buffer is given the property to deactivate the virus to avoid the risk of infection for the person taking the sample. Deactivation time is estimated at a maximum of 5 minutes.
12. How is the test transported, what is its durability and how is SAVD stored?
Everyone elements used in SAVD are freeze-dried and pre-prepared. This makes transportation very easy.
Shelf life of the various components of SAVD's test kit: 8-well SAVD strip - 6 months from production date. Positive control tube - 6 months from production date. SAVD buffer - 6 months from production date. Control buffer tube - 6 months from production date. Normalization buffer tube - 6 months from production date. Sterile swab - 3 years from production date. Sterile brush - 3 years from date of manufacture.
Temperature when storing SAVD components is as follows; for long-term storage up to 6 months, the temperature for storage is +5 to +12 degrees C. For storage up to 3 months, normal room temperature is satisfactory. However, be aware that the SAVD components must be stored in the dark and not exposed to strong sunlight. RNA is an unstable molecule and is sensitive to degradation that can be caused by, among other things. improper storage, temperature, direct sunlight or bacterial growth. According to the SAVD user manual, analysis of the test is recommended on the same day as sampling.
13. Why is high sensitivity and specificity important for a test?
The sensitivity is the probability that a sick patient will get the correct answer, i.e. a positive test. The specificity is the probability that a healthy patient will get the correct answer, i.e. a negative test. If there is a low sensitivity of a test, there is a risk of sending false negative patients, i.e. sick individuals out into society without measures. Conversely, with low specificity, one will have false positive tests, and measures such as isolation, quarantine and extensive infection tracing are done unnecessarily. SAVD has been verified with a specificity of 100% and a sensitivity of 99%.
14. What is Limit of Detection (LoD)?
Limit of Detection describes the lowest concentration that can be measured and detected by analysis. SAVD's LoD is 20 virus copies per ml. By comparison, antigen tests have a LoD of over 1 million virus copies per ml. A LoD of 20 / ml places SAVD at the top of the market.
Technical questions
A. TRANSPORT, STORAGE AND DURABILITY OF SAVD
1. How to transport the SAVD test?
All elements used in SAVD are freeze-dried and pre-prepared. This makes transportation very easy.
2. At what temperature should the components be stored?
Temperature when storing SAVD components is as follows; for long-term storage up to 6 months, the temperature for storage is +5 to +12 degrees C. For storage up to 3 months, normal room temperature is satisfactory. However, be aware that the SAVD components must be stored in the dark and not exposed to strong sunlight. RNA is an unstable molecule and is sensitive to degradation that can be caused by, among other things. improper storage, temperature, direct sunlight or bacterial growth. According to the SAVD user manual, analysis of the test is recommended on the same day as sampling.
3. How should the SAVD test be transported?
Shelf life of the various components of SAVD's test kit: 8-well SAVD strip - 6 months from production date. Positive control tube - 6 months from production date. SAVD buffer - 6 months from production date. Control buffer tube - 6 months from production date. Normalization buffer tube - 6 months from production date. Sterile swab - 3 years from production date. Sterile brush - 3 years from date of manufacture.
4. What to do in case of deviations in buffers and reagents
B. PIPETTING AND SAVD STRIPES
1. How long can it take from the pipetting is finished until the PCR machine is run?
The manufacturer GeneMe has only performed analyzes where PCR is run directly after pipetting and no later than 30 minutes after the first tube was pipetted, but it will probably also work to wait longer. The recommendation is therefore to pipette all the samples and then run PCR directly.
Can I use the SAVD strips if the structure looks different?
Yes, during transport and / or production the structure may collapse, this will change appearance, but the content and function are unchanged and the pipes can be used as usual. Perform a test using tubes with altered appearance to prepare for positive control, you will then see that the signal is unchanged.
Example of crystallized SAVD stripes:
C. CONTROL SAMPLES
1. What do you do if the positive control does not give a signal?
Check "No baseline subtraction". If the positive control is shown without an increase in RFU, then the test is invalid and must be run again using the same patient samples (ie you do not need to swab again). Remember that normalization buffer should be used to create positive control, furthermore the solution should be transferred to one of the pipes in an 8-well SAVD support. The solution should eventually turn blue (either blue immediately in the normalization buffer, or first yellow in the normalization buffer and then turn blue when mixed with the reagents in the SAVD strips).
Do we have to use two positive and two negative controls when using two different LOT numbers?
Yes. Alternatively, you can only use controls and SAVD stripes from a new LOT number and run the remnants from the old LOT number at a later time when fewer tests are to be run.
D. MACHINE DRIVING
1. How should the pipes be placed in the wells to avoid deformation (crushing) of the pipes?
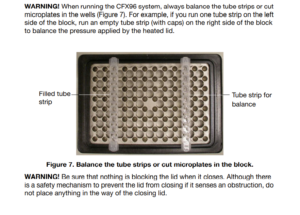
It is very important to have a balanced position and to get an even pressure when the lid on the machine is put on. For maximum even pressure, it is recommended to use support strips. Pipes located at the outer edge of the machine are more likely to be deformed, compared to pipes located further inside the well block. Any change in the condition of the plastic will affect the fluorescence signals, because it is based on reflection inside each tube. Filling the thermocycle block must be in accordance with the machine manufacturer's instructions. Visual inspection of strips after reaction to check shape and liquid level (if no evaporation has taken place), allows to eliminate unreliable results according to handling with plastic and machine. 5 (5) Place all stripes in parallel vertically, equal in number on each side, and avoid the edges. Fill all remaining wells with empty tubes supplied by GeneMe. In the illustration from machine manufacturer Bio-Rad's user manual page 9, well location is shown.
2. How are limit values adjusted and calculated?
Click here: Setting limit values
E. RESULTS
1. What is the reason for invalid result?
Invalid result occurs only if there is a positive signal in the negative control or no signal in the positive control. This only happens in the event of direct damage such as extreme heat exposure, saturation through very high humidity or the equipment being mishandled, e.g. that the plastic wells are affected without gloves and the lighting through these for analysis is most incorrect due to. dirt.
2. Click here: Guidance for analysis of samples.