
Lateral flow assay (LFA) is one of the most popular paper-based biosensors within the diagnostics industry, with a long history and significant technological advances. LFA has been developed from paper chromatography in the 1940s and adapted for various diagnostic purposes.
LFA fills the gap between diagnosis and treatment by offering advantages such as rapid response, point-of-care testing, early detection, low cost and efficient and sensitive detection of various infectious diseases as well as detection of antibodies. Paper's properties as a low-cost material with abundance, degradability and the possibility of single use have made it particularly attractive in LFA technology.
In addition to traditional visual reading methods, various readers and detection tools that provide quantitative results have been explored and developed. These readers can be used to measure and analyze the LFA results in a more accurate and objective way. Some of the readers available include fluorescence readers, photodiode array readers, portable spectrophotometers, and custom imaging devices. These reading technologies have enabled quantitative analysis and have helped increase the accuracy and reliability of LFA diagnostics.
LFA-based biosensors have proven to be very useful and have the potential to be economically successful. Although only 2% of total healthcare expenditure is spent on in vitro diagnostics, as much as 60% of treatment choices are based on this type of diagnostic data. Therefore, it is important to continue the research and development of LFA to improve the reliability and effectiveness of diagnostic tests.
The review of LFA and its development shows that this technology has a significant impact on medical diagnosis. By continuing to improve and explore new uses for LFA, including the use of quantitative readers, we can expect further advances in the field of diagnostics and better healthcare in general.
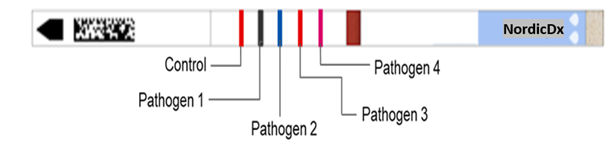
NordiDx owns the revolutionary assaya reader, which is the world's first universal solution for machine reading of rapid diagnostic tests, and the only globally approved (IVDR) medical device for in vitro diagnostics in class 1 for decentralized diagnostics.
The Assaya iaX is a smart connected device that reads test results from many different test manufacturers in seconds, and stores the results securely in the assayaDX. iaX, an IVDR approved reader is a key component of a group of products that make up the assayaDX test platform. Do not hesitate to contact us for additional information about the system and available tests.
Take a look at the attached video link.
https://nordicdx.no/en/product/iax-express/